-
Introducing
CYTISINE -
How CYTISINE
works -
Why consider
CYTISINE? -
How to use
CYTISINE - CYTISINE prescribing & safety considerations
INTRODUCING CYTISINE
- CYTISINE is indicated for smoking cessation and reduction of nicotine cravings in smokers who are willing to stop smoking 1
- The treatment goal of CYTISINE is the permanent cessation of the nicotine-containing products use 1
- The use of CYTISINE allows for a gradual reduction of nicotine dependence by relieving withdrawal symptoms 1
- CYTISINE is available in the UK as a Presciption Only Medicine (POM)
- CYTISINE has been used in Eastern Europe for smoking cessation for over 50 years 2
- CYTISINE is prescribed in a 25-day complete treatment course 1
- CYTISINE is a partial nicotine receptor agonist1
- CYTISINE Summary of Product Characteristics.
- Karnieg T, Wang X. CYTISINE for smoking cessation CMAJ 2018;190(19):E596.
Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk/. Adverse events should also be reported to Consilient Health (UK) Ltd, No. 1 Church Road, Richmond upon Thames, Surrey TW9 2QE UK or drugsafety@consilienthealth.com
HOW CYTISINE WORKS
- CYTISINE is a partial agonist of the α4β2 nicotinic acetylcholine receptor 1
- CYTISINE competes with nicotine for the same receptors and gradually displaces nicotine due to its stronger binding. It has lower ability to stimulate nicotinic receptors 1
- It prevents nicotine‑dependent full activation of the mesolimbic dopamine system and moderately increases level of dopamine in the brain. This alleviates the central symptoms of nicotine withdrawal 1
- In the peripheral nervous system, it influences breathing, catecholamine secretion, and blood pressure and helps mitigate peripheral symptoms of nicotine withdrawal 1
Mechanism of action of
nicotine dependence
cholinergic nicotinic
receptors
the dopamine
mesolimbic system

to nicotine reduces
dopamine release
resulting in withdrawal
symptoms
for nicotine
Mechanism of
action of CYTISINE
the same nicotinic receptors
high affinity for
target receptors,
CYTISINE gradually
displaces nicotine
receptors prevents full
activation of mesolimbic
dopamine system

peripheral nicotine
withdrawal symptoms
treatment, CYTISINE is
eliminated
from the body
Mechanism of action of
nicotine dependence

Mechanism of
action of CYTISINE

- CYTISINE Summary of Product Characteristics.
Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk/. Adverse events should also be reported to Consilient Health (UK) Ltd, No. 1 Church Road, Richmond upon Thames, Surrey TW9 2QE UK or drugsafety@consilienthealth.com
WHY CONSIDER CYTISINE?
- CYTISINE is indicated for smoking cessation and reduction of nicotine cravings in smokers who are willing to stop smoking. The treatment goal is the permanent cessation of the nicotine‑containing products use1
- CYTISINE has been used as a smoking cessation treatment in Eastern Europe for decades2
- CYTISINE is an effective treatment for smoking cessation; this has been shown in randomised, placebo-controlled trials3,4 and real-world use 5,6
CYTISINE OFFERS A RANGE OF BENEFITS FOR PEOPLE WHO WISH TO QUIT SMOKING1
- CYTISINE has a treatment course of 25 days 1
- CYTISINE is a partial nicotine receptor agonist 1
- CYTISINE is a Prescription Only Medicine (POM) and is available now in the UK 1
- Data from a single-centre, double-blind, parallel group trial (conducted in Poland) which included adult smokers who smoked more than 10 cigarettes a day and who were willing to attempt to stop permanently4
- Subjects (n= 740) were randomised to CYTISINE administered as 25-day treatment course or matching placebo
- Primary outcome was 12 months of abstinence after the end of treatment, defined according to the Russell Standard criteria i.e. Participants reporting:
- Fewer than five cigarettes in each of the previous 6 months at the 6-month and 12-month follow-up visits
- Not smoked any cigarettes in the week before the follow-up visit, and
-
- Carbon monoxide concentration in exhaled breath of less than 10 ppm at the 12-month follow-up visit
- Other inclusion criteria:
- not pregnant or breast-feeding or planning to become pregnant,
- willing to attend all study sessions
- able to read and write Polish and provide informed consent
- could be contacted by telephone
- Exclusion criteria
- diagnosis of a current psychiatric disorder or a medical condition that was a CYTISINE contraindication
- Baseline characteristics were evenly balanced between the treatment groups
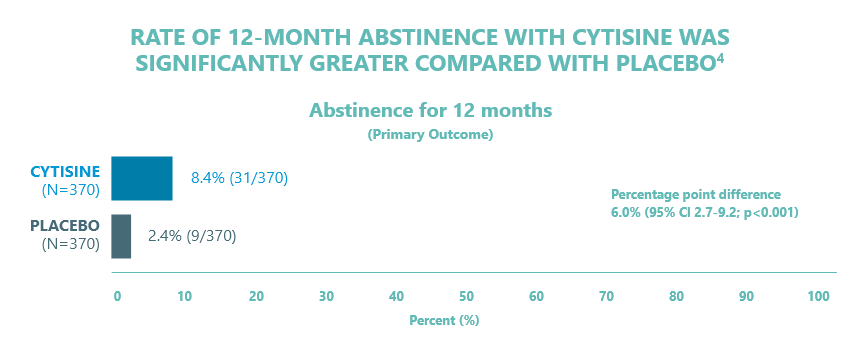
- Serious events: 7 total (4 in the cytisine group, 3 in the placebo group). Non-serious events: 203 (120 cytisine, 83 placebo). Gastrointestinal issues more common with cytisine (13.8% vs 8.1%, p=0.02)
- Data from a pragmatic, open-label, randomised, parallel group, non-inferiority trial of adult smokers motivated to quit7
- Subjects (n= 1,310) were randomised to CYTISINE or NRT. CYTISINE was posted to the subject and administered as 25-day treatment course. NRT was provided as community pharmacy vouchers for nicotine patches (in doses of 7 mg, 14 mg, or 21 mg) and for gum (2 mg or 4 mg) or lozenges (1 mg or 2 mg) or both gum and lozenges for 8 weeks.
- All subjects were offered low-intensity telephone behavioural support. CYTISINE patients were offered vouchers for NRT for use after the 25 day treatment course if they still had not stopped smoking or required ongoing support
- Primary outcome was continuous abstinence from smoking (self-reported abstinence since quit day, with an allowance for smoking a total of five cigarettes or less, including during the previous 7 days) 1 month after quit day. Self-reported continuous abstinence was also assessed at 6 months
- Inclusion criteria:
- ≥ 18 years of age, daily smokers, and motivated to quit
- Exclusion criteria:
- pregnant or breast-feeding,
- taking smoking cessation medication, enrolled in another cessation program or study,
- self-reported pheochromocytoma,
- systolic blood pressure above 150 mm Hg, a diastolic blood pressure above 100 mm Hg, or both,
- schizophrenia, or
- self-reported cardiovascular event in the 2 weeks before study enrollment
- Baseline characteristics were evenly balanced between the treatment groups

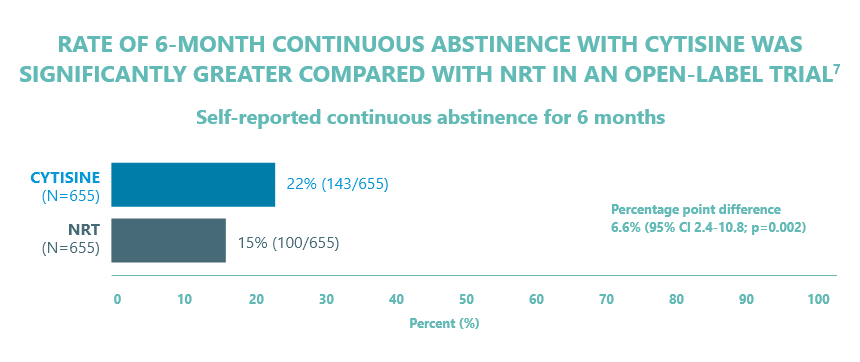
- Adverse effects were more frequent in CYTISINE group (288 events in 204 participants) than NRT group (174 events in 134 participants; incidence rate ratio: 1.7 (95% CI, 1.4 to 2.0; P<0.001); however, the majority were non-serious and mild to moderate severity.
- The most frequent in CYTISINE group: nausea, vomiting, sleep disorders.
- Serious Adverse Events were CYTISINE: 45 participants (7%). NRT: 39 participants (6%).
- CYTISINE Summary of Product Characteristics.
- Karnieg T, Wang X. CYTISINE for smoking cessation. CMAJ 2018;190(19):E596.
- Vinnikov D, Brimkulov N, Burjubaeva A. A double-blind, randomised, placebo-controlled trial of CYTISINE for smoking cessation in medium-dependent workers. J Smoking Cess 2008;3:57-62.
- West R, et al. Placebo-controlled trial of CYTISINE for smoking cessation. N Engl J Med 2011;365:1193-1200.
- Zatonski W, et al. An uncontrolled trial of CYTISINE (Tabex) for smoking cessation. Tobacco Contr 2006;15:481-484.
- Jimenez Ruiz CA, et al. The DESTINA study: An observational cross-sectional study to evaluate patient satisfaction and tolerability of CYTISINE for smoking cessation in Spain. Arch Bronconeumol 2023;59:270-72.
- Walker N, et al. CYTISINE versus nicotine for smoking cessation. N Engl J Med 2014;371:2353-62.
Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk/. Adverse events should also be reported to Consilient Health (UK) Ltd, No. 1 Church Road, Richmond upon Thames, Surrey TW9 2QE UK or drugsafety@consilienthealth.com
HOW TO USE CYTISINE?
- CYTISINE is available as a Prescription Only Medicine (POM)
- Before prescribing, please refer to the full Summary of Product Characteristics via Consilient Health website or eMC
- CYTISINE should be used in smokers who are willing to stop smoking1
- From the outset of treatment, the goal should be the permanent cessation of the nicotine-containing products use1
- CYTISINE is taken over a 25-day treatment course1
- CYTISINE tablets should be taken with a suitable amount of water according to the following schedule:
| Days of treatment | Recommended dosing | Maximum daily dose |
|---|---|---|
| From the 1st to the 3rd day | 1 tablet every 2 hours | 6 tablets |
| From the 4th to the 12th day | 1 tablet every 2.5 hours | 5 tablets |
| From the 13th to the 16th day | 1 tablet every 3 hours | 4 tablets |
| From the 17th to the 20th day | 1 tablet every 5 hours | 3 tablets |
| From the 21st to the 25th day | 1-2 tablets a day | to 2 tablets |
- Smoking should be stopped no later than on the 5th day of treatment1
- Smoking should not be continued during treatment as this may aggravate adverse reactions1
- One pack of Cytisine (100 tablets) contains enough for a complete treatment course 1
- In case of treatment failure, the treatment should be discontinued and may be resumed after 2 to 3 months 1
CYTISINE Dosing Guide and Patient Leaflet is available to download below or from Consilient
Health. Please Contact Us if you would like copies of these leaflets

- CYTISINE Summary of Product Characteristics.
Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk/. Adverse events should also be reported to Consilient Health (UK) Ltd, No. 1 Church Road, Richmond upon Thames, Surrey TW9 2QE UK or drugsafety@consilienthealth.com
CYTISINE PRESCRIBING & SAFETY CONSIDERATIONS
- Before prescribing, please refer to the full Summary of Product Characteristics via Consilient Health website or eMC
CYTISINE INDICATION 1
- CYTISINE is indicated for smoking cessation and
reduction of nicotine cravings in smokers who are willing to stop smoking - The treatment goal of CYTISINE is the permanent cessation of the nicotine-containing products use
- There is no clinical experience of CYTISINE in patients with renal or hepatic impairment, therefore CYTISINE is not recommended for use in this patient population
- CYTISINE is not recommended for use in patients <18 years and patients >65 years
CONTRAINDICATIONS 1
- Hypersensitivity to the active substance of any of the excipients (mannitol, microcrystalline cellulose, magnesium stearate, glycerol dibehenate, hypromellose)
- Unstable angina
- A history of recent myocardial infarction
- Clinically significant arrhythmia
- A history of recent stroke
- Pregnancy or breastfeeding
SPECIAL WARNINGS AND PRECAUTIONS FOR USE AND INTERACTIONS1
- Ischaemic heart disease
- Heart failure
- Hypertension
- Pheochromocytoma
- Atherosclerosis and other peripheral
vascular disease - Gastric and duodenal ulcer
- Gastroesophageal reflux disease
- Hyperthyroidism
- Diabetes
- Schizophrenia
- Depressed mood, rarely including suicidal ideation and suicide attempt, may be a symptom of nicotine withdrawal. Clinicians should be aware of the possible emergence of serious neuropsychiatric symptoms in patients attempting to quit smoking with or without treatment. History of psychiatric disorders Smoking cessation, with or without pharmacotherapy, has been associated with exacerbation of underlying psychiatric illness (e.g. depression). Care should be taken with patients with a history of psychiatric illness and patients should be advised accordingly
- Patients should be aware that the simultaneous administration of CYTISINE and smoking or use of products containing nicotine could lead to aggravated adverse reactions of nicotine
- Stopping smoking:
- Polycyclic aromatic hydrocarbons in tobacco smoke induce metabolism of drugs metabolised by CYP 1A2 (and possibly by CYP 1A1). Stopping smoking may result in slower metabolism and a rise in blood levels of such drugs. Potentially clinically important if narrow therapeutic window, e.g. theophylline, tacrine, clozapine and ropinirole
- Plasma concentration of products metabolised in part by CYP1A2 e.g. imipramine, olanzapine, clomipramine and fluvoxamine may also increase on smoking cessation; data are lacking and clinical significance unknown. Limited data indicate the metabolism of flecainide and pentazocine may also be induced by smoking
- Women of childbearing potential must use highly effective contraception while taking CYTISINE. It is currently unknown whether CYTISINE may reduce the effectiveness of systemically acting hormonal contraceptives, and therefore women using systemically acting hormonal contraceptives should add a second barrier method
- Cytisine should not be used with anti-tuberculosis drugs. No other clinical data on significant interaction with other drugs
CYTISINE TOLERABILITY 1
-
The clinical studies and previous experience with use of CYTISINE-containing product indicate a good tolerability of CYTISINE 1
-
The proportion of patients who discontinued treatment because adverse reactions was 6-15.5% and in controlled studies it was comparable to the proportion of patients who discontinued treatment in the placebo group 1
-
Mild to moderate adverse reactions have been observed with the use of CYTISINE, most frequently concerning the gastrointestinal tract 1
-
Clinical trials and prior experience indicate that the majority of adverse reactions occurred at the beginning of CYTISINE therapy and resolved during treatment 1
-
These symptoms could also be the result of smoking cessation, rather than the use of CYTISINE 1
Undesirable effects 1
| Adverse event class | Very common (≥1/10) | Common (≥1/100 to <1 /10) | Uncommon (≥ 1/1,000 to < 1/100) |
|---|---|---|---|
| Metabolism and nutrition disorders | Change in appetite (mainly increase), weight gain | ||
| Nervous system disorders | Dizziness, irritability, mood changes, anxiety, sleep disorders (insomnia, drowsiness, lethargy, abnormal dreams, nightmares), headaches | Difficulty in concentration | feeling of heaviness in the head, decreased libido |
| Eye disorders | Lacrimation | ||
| Cardiac disorders | Tachycardia | Slow heart rate | |
| Vascular disorders | Hypertension | ||
| Respiratory, thoracic and mediastinal disorders | dyspnea, increased sputum | ||
| Gastrointestinal disorders | Dry mouth, diarrhoea, nausea, changes flavour, heartburn, constipation, vomiting, abdominal pain (especially in the upper abdomen) | Abdominal distension, burning tongue | excessive salivation |
| Skin and subcutaneous tissue disorders | Rash | ||
| Musculoskeletal and connective tissue disorders | Myalgia | ||
| General disorders and administration site conditions | Fatigue | Malaise | tiredness |
| Investigations | increase in serum transaminase levels |
- CYTISINE Summary of Product Characteristics.
Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk/. Adverse events should also be reported to Consilient Health (UK) Ltd, No. 1 Church Road, Richmond upon Thames, Surrey TW9 2QE UK or drugsafety@consilienthealth.com